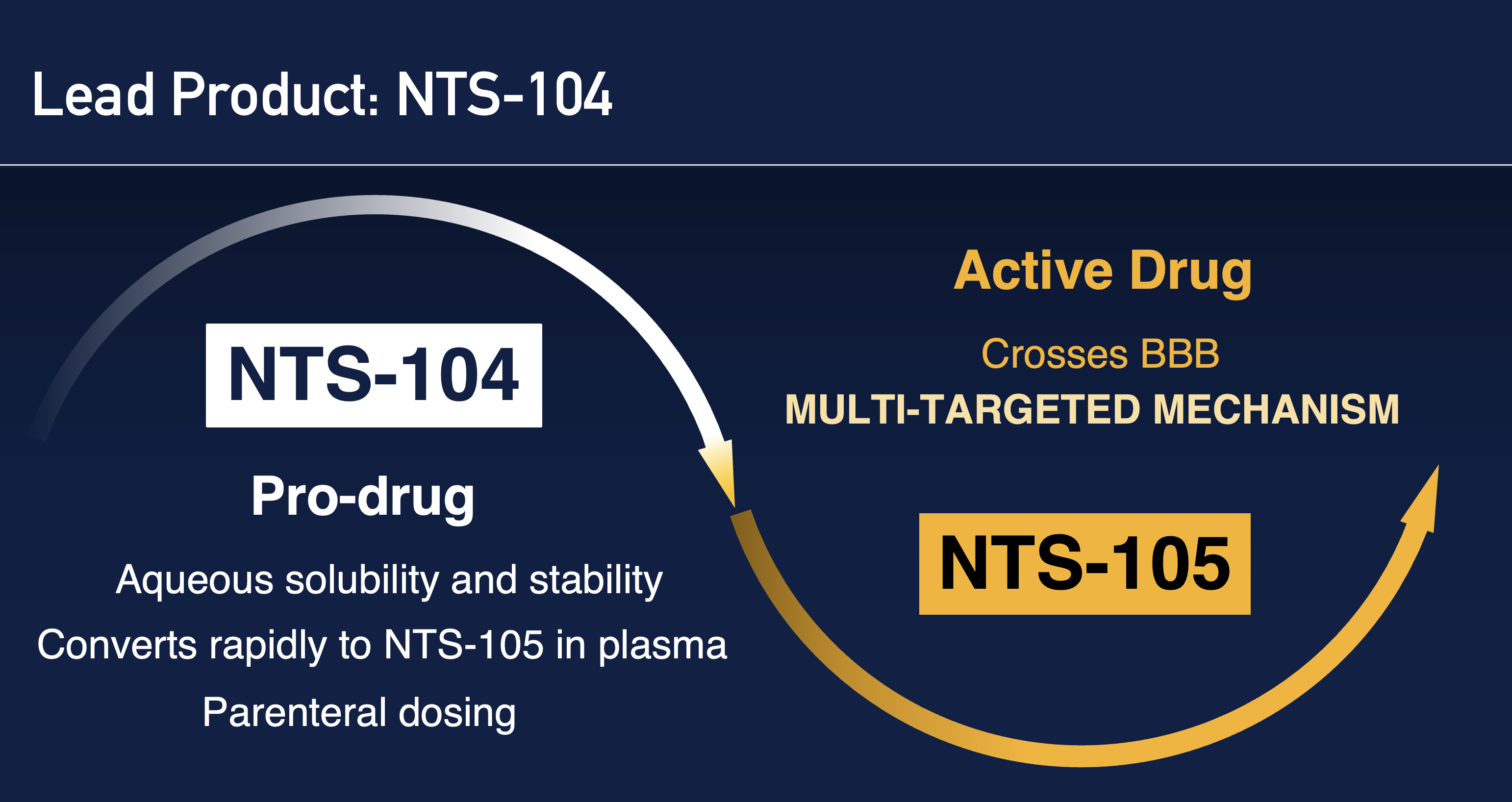
Science
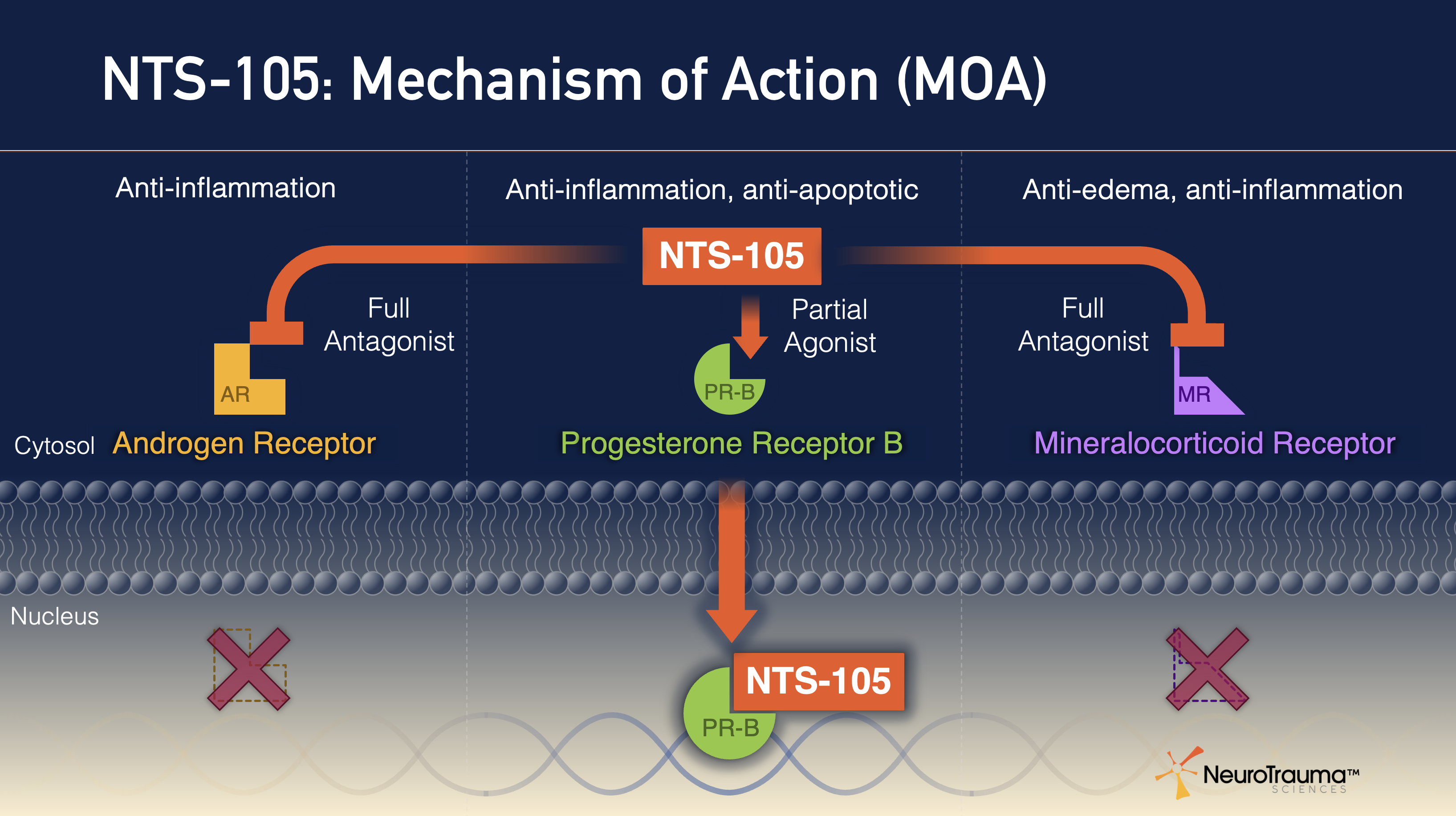
NTS-105 has a unique target receptor profile, with three equipotent receptor interactions, including:
- Full antagonist at androgen receptor
- Partial agonist to cellular progesterone receptor B
- Full antagonist at mineralocorticoid receptor
This results in potent anti-inflammatory, anti-apoptotic (inhibits cell death), and anti-edema activity in the brain and systemically.
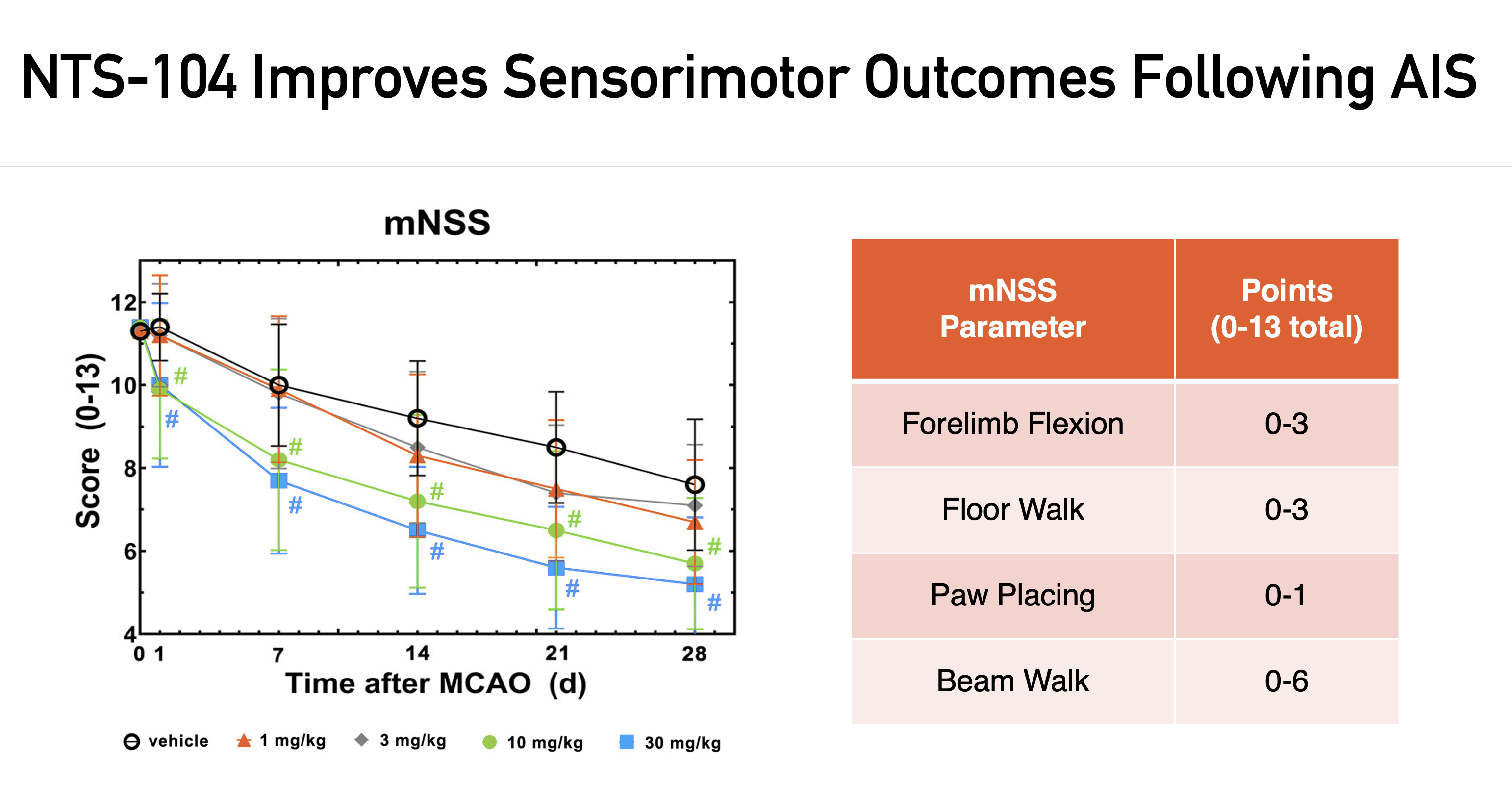
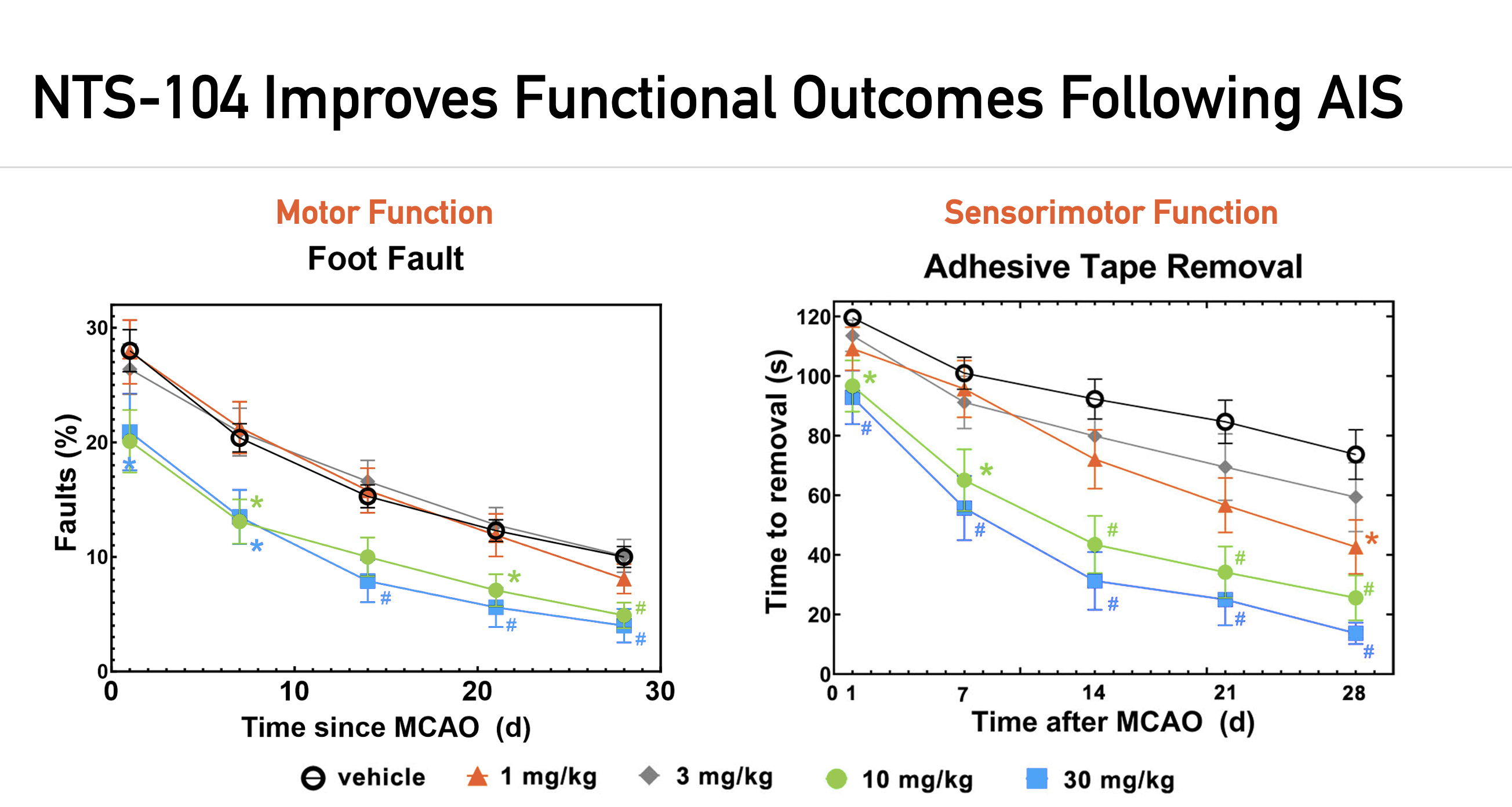
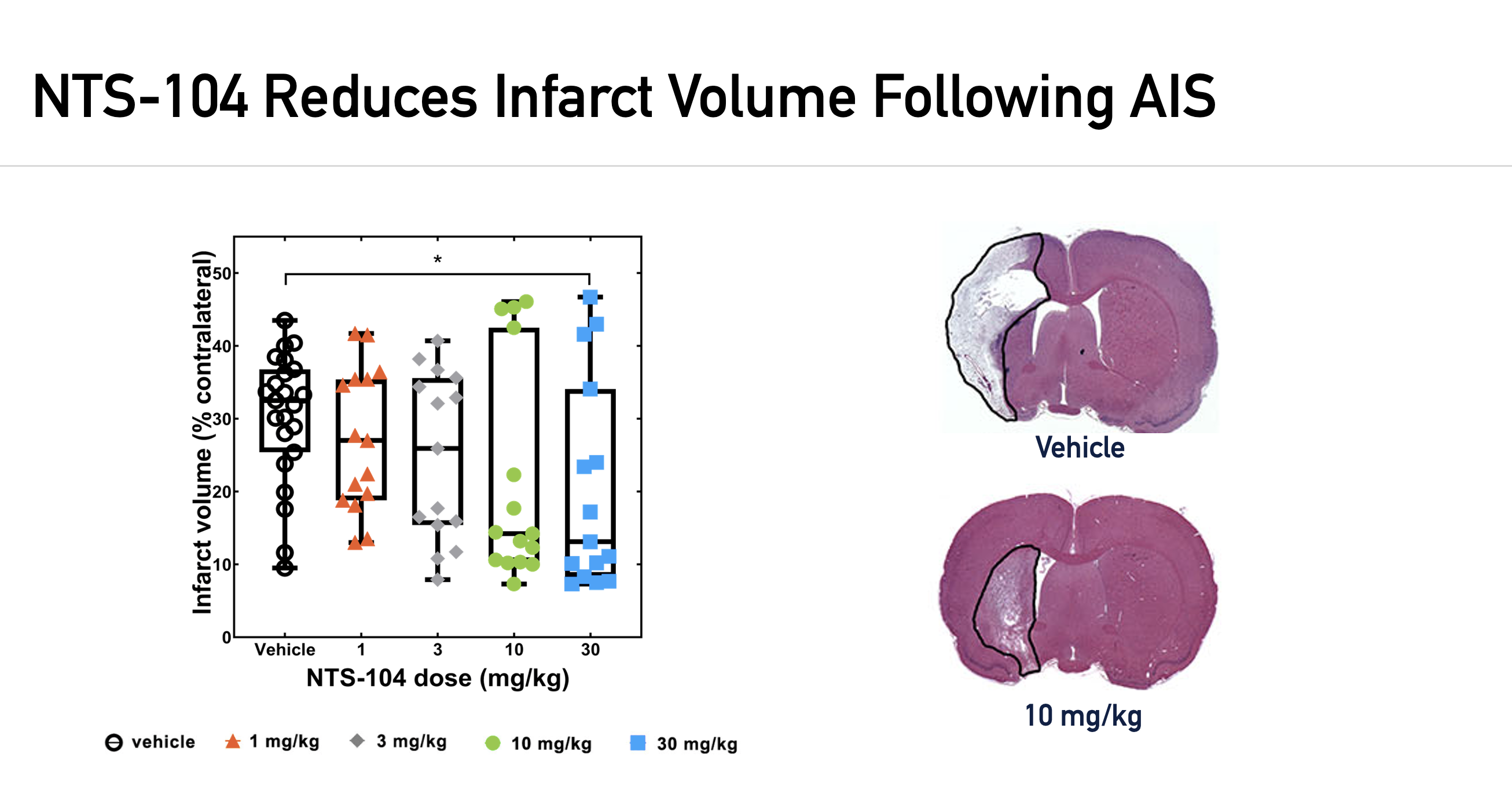
NTS has performed rigorous preclinical evaluation of NTS-104 in multiple independent laboratories utilizing two acute ischemic stroke (AIS) models, including the transient and embolic middle cerebral artery occlusion models, tMCAO and eMCAO, respectively. The following results were published in Stroke (November 2022).